Print as PDF
KEY POINTS
- Opportunistic testing of people at risk of hepatitis B virus infection should be undertaken, particularly for people born in intermediate- and high-prevalence countries, and Aboriginal and Torres Strait Islander people (1).
- Testing for hepatitis B in a patient from a hepatitis B priority population aligns with the screening provisions of the Medicare Benefits Schedule (2) and presents an opportunity to diagnose, intervene and prevent illness and death.
- Informed consent should be obtained before testing, and test results should be conveyed in a safe and culturally appropriate manner.
- When testing for hepatitis B, the tests to be ordered are: hepatitis B surface antigen (HBsAg), antibody to surface antigen (anti-HBs) and antibody to core antigen (anti-HBc). Positive HBsAg indicates current infection, positive anti-HBs indicates immunity (through vaccination or past infection), and positive anti-HBc indicates past or current infection (this test may occasionally give a false-positive result).
Click to open GESA recommendations
- Hepatitis B Consensus Statement Working Group. Australian consensus recommendations for the management of hepatitis B infection. Melbourne. Gastroenterological Society of Australia, 2022. Available at: https://www.gesa.org.au/education/clinical-information/hbv-consensus-statement/
- National HBV Testing Policy Expert Reference Committee. National hepatitis B testing policy Version 1.2 2020 [internet]. Available at: http://testingportal.ashm.org.au/hbv (last accessed 29 April 2022).
- Testing portal. Funding of HBV testing. 2020. Available at: http://testingportal.ashm.org.au/hbv/funding-of-hbv-testing(last accessed 11 April 2022)
- Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1-98.
- Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatolog. 2016;63:261-83.
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370-98.
- MacLachlan JH, Romero N, Purcell I, Cowie BC. Viral Hepatitis Mapping Project:
Hepatitis B National Report 2022. Darlinghurst, NSW, Australia: ASHM; 2024. https:// ashm.org.au/vh-mapping-project/. - Medical Board of Australia. Good medical practice: a code of conduct for doctors in Australia. March 2014. Available at: http://www.medicalboard.gov.au/Codes-Guidelines-Policies/Code-of-conduct.aspx(last accessed 20 June 2018)
- Hsu YC, Mo LR, Chang CY, Wu MS, Kao JH, Wang WL, et al. Association Between serum level of hepatitis B surface antigen at end of entecavir therapy and risk of relapse in e antigen-negative patients. Clin Gastroenterol Hepatol 2016;14:1490-8 e3.
- Brouwer WP, Chan HL, Brunetto MR, Martinot-Peignoux M, Arends P, Cornberg M, et al. Repeated measurements of hepatitis B surface antigen identify carriers of inactive HBV during long-term follow-up. Clin Gastroenterol Hepatol 2016;14:1481-9 e5.
- Marcellin P, Martinot-Peignoux M, Asselah T, Batrla R, Messinger D, Rothe V, et al. Serum levels of hepatitis B surface antigen predict severity of fibrosis in patients with e antigen-positive chronic hepatitis B. Clin Gastroenterol Hepatol 2015;13:1532-9 e1.
- Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. Part 1: Immunization of infants, children, and adolescents. MMWR Recomm Rep 2005;54(RR-16):1-31.
- Milich D, Liang TJ. Exploring the biological basis of hepatitis B e antigen in hepatitis B virus infection. Hepatology 2003;38:1075-86.
- Niederau C, Heintges T, Lange S, Goldmann G, Niederau CM, Mohr L, et al. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. New Engl J Med 1996;334:1422-7.
- Lindh M, Hannoun C. Dynamic range and reproducibility of hepatitis B virus (HBV) DNA detection and quantification by Cobas Taqman HBV, a real-time semiautomated assay. J Clin Microbiol 2005;43:4251-4.
- Weiss J, Wu H, Farrenkopf B, Schultz T, Song G, Shah S, et al. Real time TaqMan PCR detection and quantitation of HBV genotypes A-G with the use of an internal quantitation standard. J Clin Virol 2004;30:86-93.
- Chevaliez S, Dauvillier C, Dubernet F, Poveda JD, Laperche S, Hezode C, et al. The new Aptima HBV Quant Real-Time TMA assay accurately quantifies hepatitis B virus DNA from genotypes A to F. J Clin Microbiol 2017;55:1211-9.
- Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006;130:678-86.
- Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65-73.
- Australian Government Department of Health and Ageing; Medical Services Advisory Committee (MSAC). Hepatitis B virus DNA testing. March 2007. Assessment report. MSAC application 1096.
- Kau A, Vermehren J, Sarrazin C. Treatment predictors of a sustained virologic response in hepatitis B and C. J Hepatol 2008;49:634-51.
- Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology 2000;118:554-9.
- Buster EH, Hansen BE, Lau GK, Piratvisuth T, Zeuzem S, Steyerberg EW, et al. Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 2009;137:2002-9.
- Wang CC, Lim LY, Deubner H, Tapia K, Lau AW, Manansala J, et al. Factors predictive of significant hepatic fibrosis in adults with chronic hepatitis B and normal serum ALT. J Clin Gastroenterol 2008;42:820-6.
- Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol 2007;47:760-7.
- Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, et al. HBV DNA Integration and Clonal Hepatocyte Expansion in Chronic Hepatitis B Patients Considered Immune Tolerant. Gastroenterology 2016;151:986-98 e4.
- Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 2002;137:1-10.

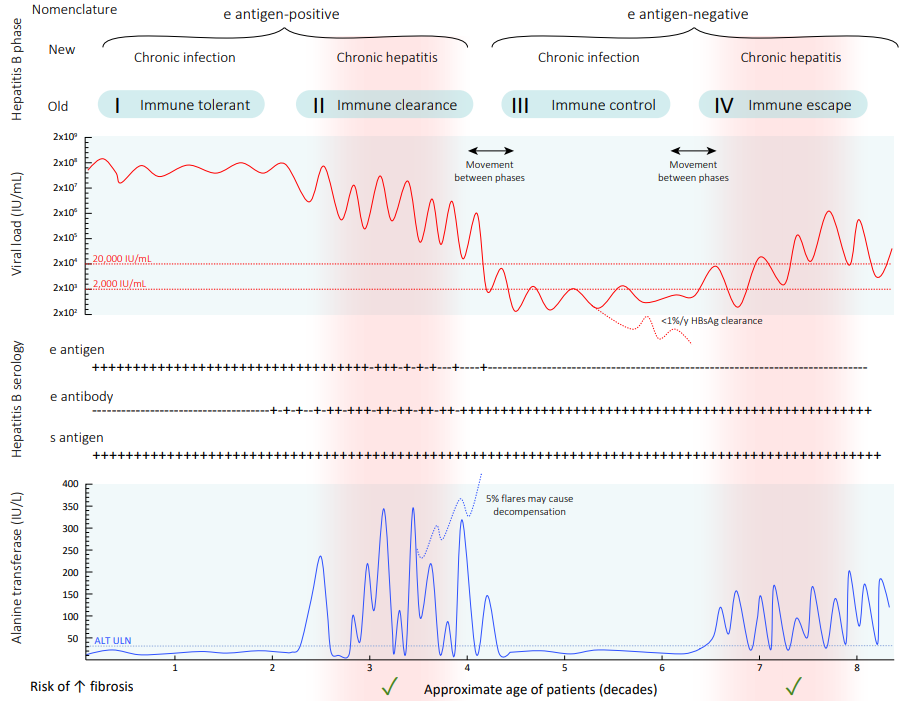 ALT = alanine aminotransferase; ULN = upper limit of normal.
ALT = alanine aminotransferase; ULN = upper limit of normal.